April 2, 2021
Converting propane into propene is expected to be more affordable and efficient
The global epidemic COVID-19 has made the consumption of masks rise sharply, therefore there is a large demand for propene.In 2020, the global propene consumption reached 116 million tons, which was equivalent to four West Lakes in Hangzhou, China.Oxidative dehydrogenation of propane (ODHP) is a key technology for producing propene from shale gas, but the challenge is that conventional metal oxide catalysts are prone to overoxidation to form valueless Cox.
The research group led by Prof. XIAO Feng-Shou and WANG Liang from Zhejaing University College of Chemical and Biological Engineering (ZJU CBE) has developed a more dynamic and endurance catalyst, which is expected to make propene production cheaper and more efficient.They found that the isolated boron in a zeolite framework without such oligomers exhibits high activity and selectivity for ODHP, which also hinders full hydrolysis for boron leaching in a humid atmosphere because of the B–O–SiOx linkage, achieving superior durability in a long-period test. A related study (Isolated boron in zeolite for oxidative dehydrogenation of propane) has recently been published in the Science.
The group used a more common zeolite molecular sieve material. In this structure, there are silico species around boron which are isolated rather than poly boron. The group was surprised that the catalyst with specific coordination environment boron center has excellent catalytic performance in the oxygen dehydrogenation of propane, which is far beyond the traditional supported boron oxide catalyst. Isolated boron is the suitable catalyst for ODHP.
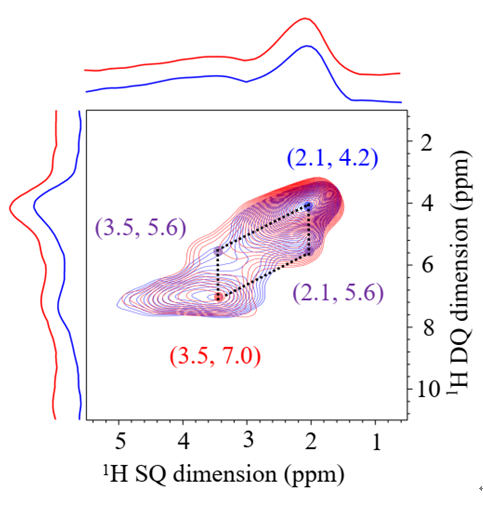
“I was not sure at first, for so many people said that isolated boron is not good." XIAO Feng-Shou said. The research group and other partners confirmed that boron exists in the framework of zeolite in isolation.
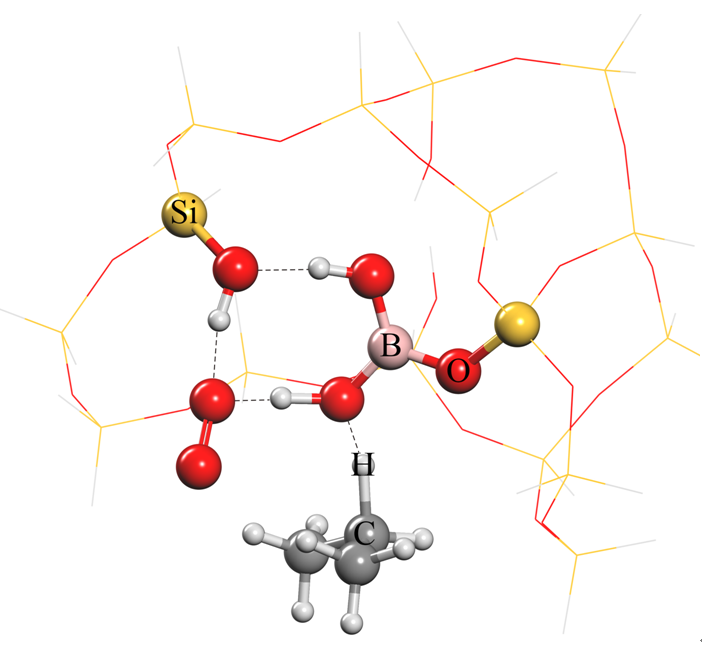
The researchers further explained the reason for the excellent catalytic performance: in the propane dehydrogenation reaction, boron dihydroxy and one of the silicon hydroxyl can activate propane and oxygen molecules at the same time, forming a stable intermediate and further converting to propene.
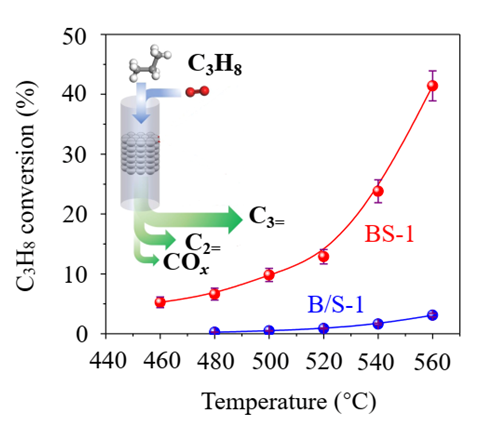
In a continuous 220-hour “endurance” test, this zeolite-catalytic oxidative dehydrogenation process maintained a high selectivity of 83% and achieved a conversion rate of 32.9-43.7%. This work breaks conventional knowledge that isolated boron centers cannot catalyze propane dehydrogenation reactions and further deepens the understanding of propane dehydrogenation and its active centers. It represents a large stride forward in boron catalysis that may lead the way to scalable applications in ODHP.
To learn more about this paper,go to https://science.sciencemag.org/content/372/6537/76.
To learn more about Professor XIAO Feng-Shou , go to https://person.zju.edu.cn/en/fsxiao.
To learn more about ZJU 100 Young Professor WANG Liang , go to https://person.zju.edu.cn/en/liangwang.

