Cancer remains the second leading cause of both incidence and mortality among humans worldwide, and China has felt its devastating impact most acutely. In 2022, the country recorded 4.82 million new cancer cases and 2.57 million cancer-related deaths—the highest figures globally. This overwhelming prevalence of cancer has fueled widespread fear among the public. One of the most promising approaches to combat cancer is tumor-targeted drug delivery, which aims to precisely transport anti-tumor drugs directly to cancer cells. However, traditional drug delivery systems have struggled with low targeting efficiency, limiting the overall efficacy of such treatments.

Over the past 15 years, a research team led by Professor SHEN Youqing from the College of Chemical and Biological Engineering at Zhejiang University has been committed to tackling these limitations. Starting by designing and synthesizing medical functional polymers, they discovered a novel tumor-active drug delivery mechanism by which the tumor itself actively ‘demands’ the drug. On this basis, they developed an intelligent drug delivery system that addresses two key challenges: ‘difficulty in exiting blood vessels’ and ‘difficulty in penetrating tumor tissues.’ This innovation has significantly improved the efficacy of targeted therapies.In recognition of this achievement, Professor Shen’s team was awarded the Second Prize of the 2023 National Natural Science Award.
Innovative mechanism: Breaking two major bottlenecks
For years, researchers in tumor-targeted drug delivery relied on a theory derived from small animal tumor models, which suggested that tumor capillaries had many pores, allowing drug delivery systems to 'leak' into tumors and accumulate there—an effect known as the Enhanced Permeability and Retention (EPR) effect. This theory formed the basis for designing many drug delivery systems. However, when tested in clinical practice, a significant challenge emerged: human tumor blood vessels have few pores, rendering it difficult for these systems to ‘leak out’ of the bloodstream and penetrate tumor tissues.
Adding to this issue, these drug delivery systems also face a second barrier—struggling to diffuse into the deep areas of tumors far from the blood supply. These dual challenges—difficulty in exiting the blood vessels and difficulty in penetrating tumor tissues—have long remained unresolved in tumor-targeted drug delivery, greatly limiting the effectiveness of the drugs.
But now, the researchers have shifted their focus from the traditional passive diffusion model to a novel approach: can cancer cells be ‘coaxed’ into actively transporting drugs?
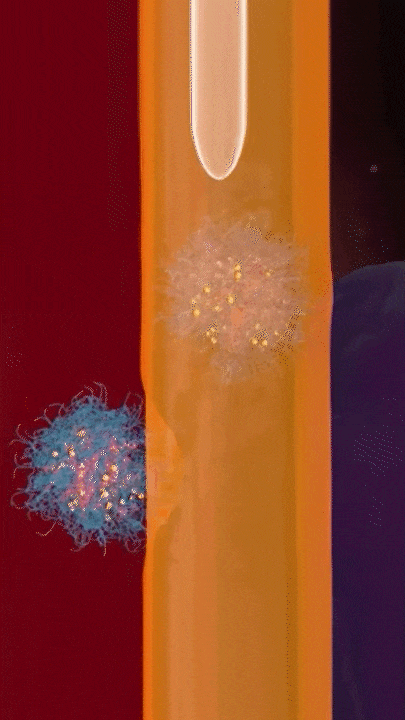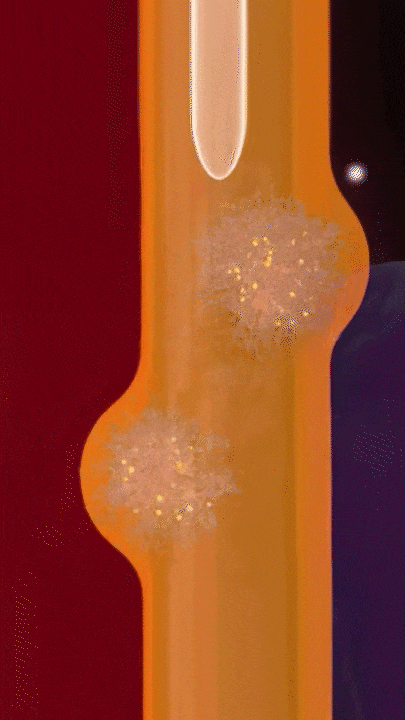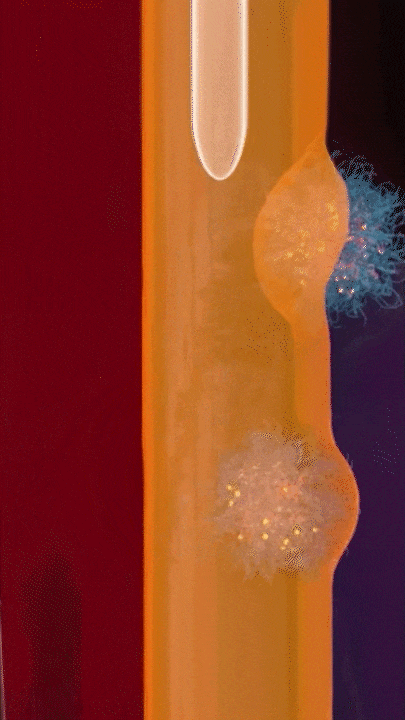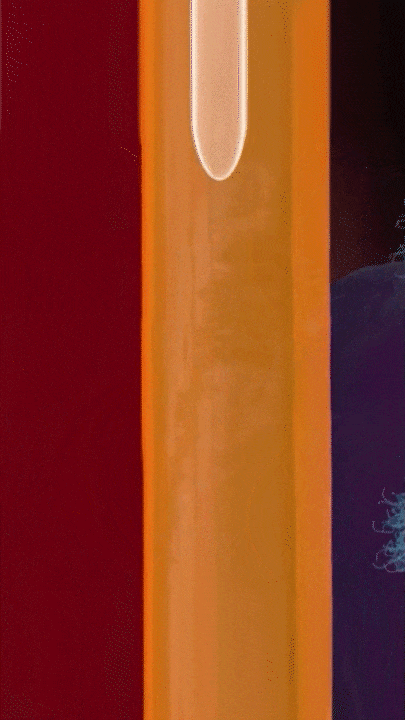
This breakthrough came when the research team discovered that positively charged polymers could trigger a cellular process called transcytosis, where tumor cells actively engulf polymer-based drug carriers and expel them out from other locations. This discovery led to the development of a new drug delivery mechanism that that essentially activates tumor cells to deliver drugs. Instead of passively relying on diffusion, tumor cells now grab the drug delivery system from the bloodstream and then share the drugs with other nearby tumor cells. This allows the drug to penetrate deep into tumor tissues, greatly enhancing its ability to eliminate cancer cells.
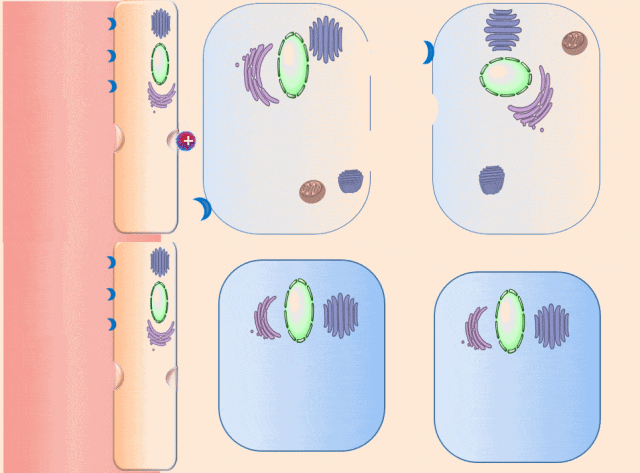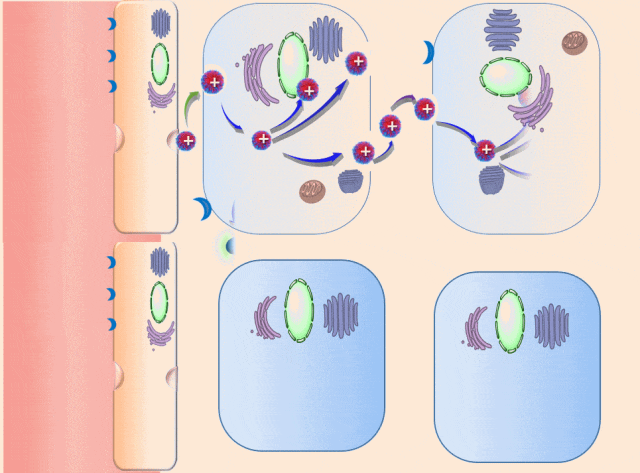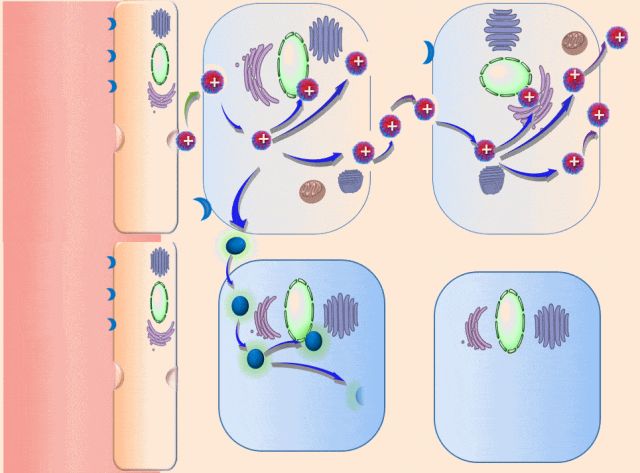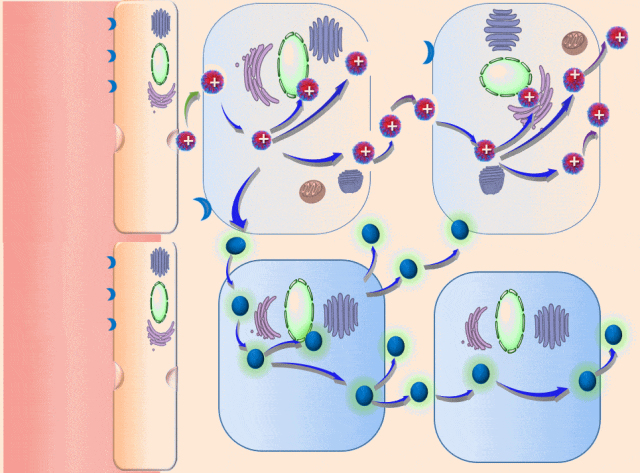
Building on this discovery, the team synthesized enzyme-responsive polymers, specifically designed for tumors with high levels of gamma-glutamyl transferase, such as pancreatic cancer. These polymers remain electrically neutral or negatively charged in the blood, but upon entering the tumor environment, they can rapidly switch to a positive charge promoting the quick ‘relay’ transportation of the drug delivery system across cells.
Comprehensive design: Building an efficient drug delivery system
The team has also been dedicated to developing advanced polymer drug delivery materials, laying the foundation for large-scale production. Clear carrier structures, non-toxicity, and scalable production are vitally essential for clinical translation and application. Among the promising candidates for drug delivery are dendritic polymers, which are known for their well-defined structure and non-toxic properties. However, challenges such as low preparation efficiency, purification difficulties, and limited scalability have hindered their broader use.
To overcome these obstacles, the team has developed a novel synthesis method for dendritic polymers. Using asymmetric monomers in an orthogonal double-click reaction, they have streamlined the process, improving both efficiency and yield while ensuring structural precision. Remarkably, this new technique enables the synthesis of Generation 5 dendritic polymers in just one day—a significant leap forward for the field.
Building on this advancement, the team has constructed an efficient drug delivery system using carrier materials. They have formulated a five-step cascade process termed as a CAPIR (circulation, accumulation, penetration, internalization and release) cascade, which includes circulation in the blood compartments, accumulation in the tumor, deep penetration into avascular tumor tissue, cellular internalization and intracellular drug release. This process addresses the complex and sometimes conflicting requirements of each step in the drug delivery system. By leveraging the adaptive properties of the carrier, the team has proposed the principles for integrating and coordinating the functions of the drug delivery system and developed corresponding methods for controlling carrier properties, resulting in a functionally integrated system.
This innovative approach has led to three major breakthroughs in translational medicine. One of the achievements has progressed to clinical trials in the United States and South Korea and obtained the Orphan Drug status in the United States. The other two have secured clinical trial approvals in China and the United States, offering a beacon of hope to patients battling cancers such as pancreatic, lung, and colorectal cancer.

“Our team will delve deeper into the relationship between polymer structure and transcytosis capability,” said Professor Shen. “We aim to develop more precise synthesis methods for functionally integrated polymers, and build even more efficient drug delivery systems to provide powerful new tools in the fight against cancer.”
Source:15 years in the making: ZJU researchers ‘forge’ a powerful weapon against cancer

